UFABET เว็บเดิมพันออนไลน์ครบวงจร อันดับ 1 มาตรฐานระดับโลก
UFABET (ยูฟ่าเบท) เป็นเว็บพนันออนไลน์ที่ได้รับความนิยมอย่างต่อเนื่อง ทั้งในประเทศไทยและต่างประเทศ จากข้อมูลของ SimilarWeb และ Google Trends ระบุว่า ยูฟ่าเบทไทย เป็นหนึ่งในเว็บไซต์ที่มีจำนวนผู้ใช้งานจริงและยอดค้นหาสูงสุดในกลุ่มเว็บเดิมพันออนไลน์ ซึ่งสะท้อนให้เห็นถึงความเชื่อมั่นและการยอมรับจากผู้เล่นในหลายประเทศทั่วเอเชีย
ในภูมิภาคเอเชียตะวันออกเฉียงใต้ UFABET เว็บตรง ยังติดกลุ่มเว็บไซต์ที่มีผู้ใช้งานซ้ำ (Retention Rate) สูงกว่าค่าเฉลี่ยอุตสาหกรรมกว่า 35% ปัจจัยสำคัญมาจากระบบที่ออกแบบภายใต้แนวคิด Infrastructure-Centric Design ซึ่งมุ่งเน้นความเสถียร ความปลอดภัย และการจัดการภายในที่แข็งแรง เพื่อให้ผู้เล่นสามารถใช้งานได้อย่างต่อเนื่องโดยไม่สะดุด
UFABET เว็บเดิมพันครบวงจร รวมเกมทุกประเภทไว้ในระบบเดียว ครอบคลุมทั้ง กีฬา คาสิโน สล็อต และหวยออนไลน์ ผู้เล่นสามารถเลือกใช้งานได้อย่างราบรื่นภายใต้บัญชีเดียว ไม่ต้องย้ายระบบไปมาให้ยุ่งยาก
บริการหลักของ ยูฟ่าเบท
แทงบอลออนไลน์ ด้วยอัตราต่อรองที่ชัดเจน ระบบคำนวณผลอัตโนมัติ รวดเร็วและแม่นยำ
คาสิโนสด ถ่ายทอดสดจากสถานที่จริง ภาพคมชัดระดับ HD ให้บรรยากาศสมจริง
สล็อตออนไลน์ แสดงค่า RTP (Return to Player) อย่างโปร่งใส มีเกมให้เลือกจากหลายค่าย
หวยออนไลน์ ระบบทันสมัย ตรวจสอบผลได้รวดเร็ว ปลอดภัย และแม่นยำ
ด้วยโครงสร้างระบบที่ครอบคลุมในทุกด้าน ทำให้ เว็บพนันออนไลน์ UFABET กลายเป็นศูนย์กลางของความบันเทิงและการลงทุนบนโลกดิจิทัล ที่ทั้งผู้เล่นทั่วไปและผู้ที่ต้องการสร้างรายได้อย่างจริงจังให้ความไว้วางใจอย่างต่อเนื่อง
ยูฟ่าเบท แบรนด์ชั้นนำที่ออกแบบระบบด้วยมุมมองของผู้เล่นเป็นหลัก
ยูฟ่าเบท (UFABET) หรือที่หลายคนเรียกกันสั้น ๆ ว่า UFA ไม่ได้เป็นเพียงเว็บไซต์เดิมพันออนไลน์ทั่วไป แต่ถูกพัฒนาให้เป็นระบบระดับ Enterprise-Grade ที่ให้ความสำคัญกับ User Experience หรือประสบการณ์ผู้ใช้งานในทุกจุดของการใช้บริการ
สิ่งที่ทำให้ ยูฟ่าเบท กลายเป็นชื่อที่ได้รับความไว้วางใจในระดับเอเชีย คือการดำเนินงานในรูปแบบ ยูฟ่าเบทเว็บตรง ซึ่งทุกขั้นตอน ตั้งแต่การฝาก–ถอน ไปจนถึงการเดิมพัน ดำเนินการผ่าน ระบบอัตโนมัติ (Auto System) ที่ให้ความสำคัญกับความปลอดภัย โปร่งใส และตรวจสอบได้จริง
ผู้ใช้งานจึงมั่นใจได้ว่าทุกกระบวนการอยู่ภายใต้มาตรฐานเดียวกันทั้งหมด ไม่มีการแทรกแซงหรือปรับแต่งผลการเล่น นอกจากนี้ ยังสามารถตรวจสอบรายละเอียดต่าง ๆ ได้จาก รีวิวเว็บพนันออนไลน์ UFABET ที่ได้รับการจัดอันดับจากหลายเว็บไซต์คุณภาพทั่วภูมิภาค ซึ่งสะท้อนถึงความน่าเชื่อถือของแบรนด์ในมุมมองของผู้เล่นจริง
เหตุผลที่ทำให้ ยูฟ่าเบท เว็บตรง เป็นชื่อที่ผู้เล่นให้ความเชื่อถือมายาวนาน

ยูฟ่าเบท เว็บตรง ดำเนินงานภายใต้ระบบ UFABET Company
ยูฟ่าเบทเว็บตรง เป็นผู้ให้บริการที่จัดการระบบด้วยรูปแบบ UFABET Company ซึ่งเน้นความโปร่งใสและความน่าเชื่อถือ ผู้เล่นสามารถควบคุมขั้นตอนต่าง ๆ ได้ด้วยตนเองแบบ Real-Time ทั้งการฝาก–ถอน รวมถึงการตั้งค่าบัญชีโดยไม่ต้องผ่านตัวกลาง ทำให้กระบวนการทั้งหมดเป็นไปอย่างรวดเร็ว ปลอดภัย และยุติธรรม

ระบบเสถียรภาพสูง รองรับการใช้งานอย่างต่อเนื่อง
โครงสร้างของ UFA เว็บตรง ถูกพัฒนาด้วยเทคโนโลยี Streaming Engine ที่มีมาตรฐานระดับองค์กร ช่วยให้ระบบรองรับผู้เล่นจำนวนมากได้พร้อมกันโดยไม่เกิดปัญหาความหน่วงหรือล่าช้า ไม่ว่าจะเป็นการเล่น คาสิโนสด หรือ เดิมพันกีฬา ผู้ใช้สามารถใช้งานได้อย่างราบรื่นและมั่นใจในคุณภาพของสัญญาณตลอดเวลา

ความปลอดภัยคือหัวใจสำคัญของระบบ
เว็บไซต์ UFA BET ใช้เทคโนโลยี เข้ารหัสข้อมูลขั้นสูง (Encryption System) ร่วมกับการดูแลโดยทีม Security Operation Center (SOC) ตลอด 24 ชั่วโมง เพื่อป้องกันการรั่วไหลของข้อมูลและการเข้าถึงโดยไม่ได้รับอนุญาต ทุกธุรกรรมภายในระบบจึงอยู่ภายใต้การควบคุมอย่างรัดกุมและได้มาตรฐานระดับองค์กร

ฐานผู้เล่นทั่วเอเชียและการยอมรับระดับสากล
UFA BET ได้รับการยอมรับจากผู้เล่นในหลายประเทศ เช่น ไทย ญี่ปุ่น เวียดนาม อินโดนีเซีย จีน รวมถึงบางประเทศในยุโรป การเติบโตของฐานผู้ใช้งานเหล่านี้สะท้อนให้เห็นถึงความน่าเชื่อถือและมาตรฐานของระบบบริการ ที่ได้รับการพัฒนาให้สอดคล้องกับความต้องการของผู้เล่นจากหลากหลายภูมิภาคอย่างแท้จริง
ระบบเดิมพันออนไลน์ที่ออกแบบเพื่อผู้เล่นในเอเชียและต่างประเทศ
ยูฟ่า เบท พัฒนาขึ้นจากการเข้าใจพฤติกรรมและรูปแบบการเล่นของผู้ใช้งานในเอเชีย พร้อมคงมาตรฐานระดับสากลในทุกขั้นตอน ระบบถูกออกแบบให้รองรับหลายภาษา ทั้ง ไทย อังกฤษ จีน ญี่ปุ่น และเวียดนาม เพื่อให้ผู้เล่นจากหลายประเทศสามารถใช้งานได้อย่างสะดวกและมีรูปแบบที่เป็นหนึ่งเดียวกัน
ทีมบริการลูกค้า ยูฟ่าซัพพอร์ต (UFA Support) พร้อมดูแลตลอด 24 ชั่วโมง ด้วยการตอบกลับที่รวดเร็วและเข้าใจปัญหาของผู้ใช้จริงอย่างตรงจุด ทีมงานทุกคนผ่านการฝึกอบรมเฉพาะด้านการบริการ ทำให้สามารถให้คำแนะนำได้อย่างถูกต้องและช่วยแก้ไขปัญหาได้ทันที เพื่อให้การใช้งานเป็นไปอย่างต่อเนื่องโดยไม่สะดุด
สำหรับผู้เล่นชาวไทย เว็บยูฟ่าเบท ได้รับการยอมรับว่าเป็นเว็บไซต์ที่ใช้งานง่ายและมีความน่าเชื่อถือสูง ระบบการเงินรองรับทุกธนาคารภายในประเทศ รวมถึงการชำระผ่าน QR Code Payment ที่ทำให้การ ฝาก–ถอนออโต้ เสร็จสิ้นภายในเวลาไม่ถึงหนึ่งนาที เพิ่มทั้งความสะดวกและความมั่นใจให้กับผู้เล่นในทุกธุรกรรม
Sports Book เดิมพันกีฬา
UFABET แทงบอลออนไลน์ ราคาบอลดีที่สุด ครบทุกลีกจากทั่วโลก
เว็บบอลยูฟ่าเบท ได้รับการจัดอันดับให้เป็นหนึ่งในเว็บไซต์ แทงบอลเว็บตรง ที่มีผู้ใช้งานมากที่สุดในภูมิภาคเอเชีย
ข้อมูลจาก Asia Gambling Index ล่าสุดระบุว่า ยูฟ่าเบทมีอัตราการใช้งานซ้ำ (Retention Rate) เฉลี่ยสูงถึง 74% และให้ ค่าน้ำ 4 ตังค์ ซึ่งถือว่าดีกว่ามาตรฐานของตลาดทั่วไปอย่างชัดเจน ส่งผลให้ผู้เล่นได้รับผลตอบแทนที่คุ้มค่ากว่าการเดิมพันผ่านเว็บทั่วไป
ไม่ว่าจะเป็นผู้เล่นที่เน้นวิเคราะห์ข้อมูลหรือผู้ที่ต้องการแทงบอลอย่างมีระบบ UFA ได้ออกแบบเครื่องมือและระบบการใช้งานให้ครอบคลุมทุกขั้นตอนของการเดิมพันฟุตบอลออนไลน์ โดยมีฟังก์ชันหลักที่ช่วยให้การแทงบอลมีความแม่นยำและมีประสิทธิภาพมากขึ้น เช่น
ตารางแข่งขันสด (Live Match Schedule) ที่อัปเดตข้อมูลแบบเรียลไทม์
ค่าน้ำแบบ Dynamic Odds ปรับเปลี่ยนตามสถานการณ์ของเกมจริง
ข้อมูลสถิติก่อนเกม ที่ตรวจสอบได้และเชื่อถือได้สูง
ระบบถ่ายทอดสด (Live Stream) ที่ซิงก์กับราคาต่อรองแบบ Real-Time อย่างแม่นยำ
สำหรับผู้ที่ต้องการเข้าใจว่า “แทงบอลออนไลน์ ยูฟ่าเบท คืออะไร และเหมาะกับใคร”
การเดิมพันรูปแบบนี้คือการทายผลการแข่งขันฟุตบอลผ่านระบบออนไลน์ โดย UFA BET ได้พัฒนาระบบให้การแทงบอลไม่ใช่เพียงการคาดเดาผล แต่เป็นกระบวนการวิเคราะห์ข้อมูลจริง การใช้สถิติ และการวางแผนเชิงกลยุทธ์ เพื่อเพิ่มความแม่นยำและสร้างโอกาสในการทำกำไรอย่างมีหลักการ
แทงบอล UFABET ครอบคลุมทุกลีก ทั้งในและต่างประเทศ
หนึ่งในเหตุผลที่ทำให้ ยูฟ่าเบท (UFABET) กลายเป็นเว็บ แทงบอลออนไลน์ ชั้นนำในเอเชีย คือความครบถ้วนของรายการแข่งขันที่เปิดให้เดิมพันจากทั่วโลก ไม่ว่าจะเป็นลีกชั้นนำในยุโรป เช่น Premier League, La Liga, Serie A, Bundesliga และ UEFA Champions League หรือแม้แต่ลีกในเอเชียอย่าง J-League, Thai League, K-League รวมถึงลีกจากตะวันออกกลาง ก็มีให้เลือกเล่นอย่างต่อเนื่อง
ระบบของ UFABET เว็บตรง ได้รับการออกแบบให้เชื่อมต่อข้อมูลการแข่งขันแบบ เรียลไทม์ พร้อม ตารางแข่งขันสด (Live Match Schedule) ที่อัปเดตตลอด 24 ชั่วโมง รวมถึงการแสดงผล Dynamic Odds หรือค่าน้ำที่ปรับเปลี่ยนตามสถานการณ์จริงของเกมอย่างแม่นยำ ช่วยให้ผู้เล่นสามารถวิเคราะห์และเลือกวางเดิมพันได้อย่างมีประสิทธิภาพในทุกลีกการแข่งขันทั่วโลก
แทงบอลสดแบบเรียลไทม์ ด้วยระบบ Live Betting มาตรฐานสากล
ระบบ แทงบอลสด (Live Betting) ของ UFABET ถูกออกแบบให้ทำงานประสานกับระบบ ถ่ายทอดสดแบบ Live Streaming Sync ทำให้ผู้เล่นสามารถดูการแข่งขันและวางเดิมพันได้ในเวลาเดียวกัน โดยราคาบอลจะอัปเดตแบบ Real-Time ตามจังหวะเกมจริงในสนาม
ข้อมูลสำคัญที่เกี่ยวข้องกับการแข่งขัน เช่น สถิติการครองบอล, จำนวนการยิงตรงกรอบ, และ จำนวนฟาวล์สะสมถูกแสดงให้เห็นอย่างละเอียดเพื่อช่วยให้การวิเคราะห์เกมแม่นยำยิ่งขึ้น การผสานกันของข้อมูลสดและระบบถ่ายทอดสดที่เสถียร ทำให้การ แทงบอลสด มีทั้งความยุติธรรม ความโปร่งใส และความต่อเนื่องในการใช้งานตลอดการแข่งขัน
เลือกแทงบอลเต็งหรือสเต็ป แบบไหนคุ้มค่ากว่ากัน
- บอลเต็ง (Single Match Betting)
เหมาะสำหรับผู้เล่นที่ชอบวิเคราะห์เกมอย่างละเอียดในคู่เดียว เน้นความแม่นยำและการควบคุมเงินเดิมพันได้ชัดเจน
ลักษณะการแทงแบบนี้จบเกมไว ผลได้–เสียรู้ทันที เหมาะกับผู้ที่ต้องการลดความเสี่ยงและติดตามผลแบบทันสถานการณ์
- บอลสเต็ป (Mix Parlay)
เป็นการเลือกเดิมพันหลายคู่ในบิลเดียว ซึ่งแม้จะมีความเสี่ยงมากกว่า แต่ผลตอบแทนก็สูงขึ้นตามจำนวนคู่ที่เลือก
ผู้เล่นที่เข้าใจรูปแบบทีม สถิติ และสภาพการแข่งขันในแต่ละลีก จะได้เปรียบในการคัดคู่ที่มีโอกาสทำกำไรสูง
การเล่นบอลสเต็ปจึงเหมาะกับผู้ที่มีความรู้ด้านฟุตบอลและต้องการผลกำไรที่มากขึ้นในหนึ่งรอบการเดิมพัน
ปรโมชันและกิจกรรมพิเศษจากเว็บแทงบอลยูฟ่าเบท
หนึ่งในจุดเด่นของ UFA BET เว็บตรง คือระบบผลตอบแทนที่มอบความคุ้มค่าให้กับผู้เล่นอย่างต่อเนื่อง
โดยเว็บไซต์มีการ คืนค่าคอมมิชชัน 0.5% ทุกยอดเดิมพัน ไม่ว่าผลการแข่งขันจะออกมาอย่างไร ผู้เล่นยังได้รับผลตอบแทนกลับคืนในทุกบิล
นอกจากนี้ยังมี โปรโมชันพิเศษสำหรับสมาชิกใหม่, กิจกรรมเติมเงินรายวัน และโบนัสสะสมที่อัปเดตตลอดทั้งเดือน
ทั้งหมดนี้ช่วยเพิ่มมูลค่าให้กับการเล่นในทุกครั้ง และสะท้อนแนวทางของ ยูฟ่าเบท (UFABET) ที่ต้องการให้ผู้ใช้งานได้รับประโยชน์สูงสุดจากทุกการเดิมพัน
ตาราง ราคาบอล เว็บบอลยูฟ่า ครอบคลุมทุกคู่ ทุกประเภทการเล่น
ประเภทราคา (Odds Format) | ตัวอย่างค่าน้ำ | วิธีคำนวณผลตอบแทน |
| ค่าน้ำมาเลย์ (MY) | -0.95 / 0.80 | แทง 100 หากแพ้เสีย 95 (ค่าน้ำแดง), หากชนะได้ 80 (ค่าน้ำดำ) |
| ค่าน้ำฮ่องกง (HK) | 0.95 / 1.20 | แทง 100 ได้กำไร 95 หรือ 120 ไม่รวมทุน |
| ค่าน้ำยุโรป (EU) | 1.95 / 2.20 | แทง 100 ได้คืน 195 หรือ 220 รวมทุน |
| ค่าน้ำอินโดนีเซีย (ID) | -1.05 / 1.25 | ลักษณะคล้ายค่าน้ำมาเลย์ แต่กลับทิศทางของการคำนวณ |
| ค่าน้ำอเมริกัน (US) | -105 / +125 | ค่าน้ำ -105 หมายถึง แทง 105 ได้ 100 ส่วน +125 หมายถึง แทง 100 ได้ 125 |
หมายเหตุเพิ่มเติม:
ยูฟ่าเบท รองรับค่าน้ำครบทุกประเภท ผู้เล่นสามารถเลือกใช้งานตามความถนัดได้จากหน้าเดิมพันโดยตรง ซึ่งระบบจะคำนวณผลตอบแทนให้แบบอัตโนมัติทันทีหลังจบการแข่งขัน
Casino Online (คาสิโน)
คาสิโนออนไลน์ UFABET ถ่ายทอดสดสตูดิโอจริง เล่นง่ายทุกอุปกรณ์
ในช่วงไม่กี่ปีที่ผ่านมา วงการ คาสิโนออนไลน์ เติบโตขึ้นอย่างก้าวกระโดด โดยรูปแบบที่ได้รับความนิยมมากที่สุดคือ คาสิโนสด (Live Casino) ซึ่งผสมผสานความสะดวกของระบบออนไลน์กับความสมจริงของบ่อนจริงได้อย่างลงตัว
สำหรับผู้เล่นชาวไทย UFA CASINO ถือเป็นหนึ่งในชื่อที่ได้รับการยอมรับอย่างกว้างขวาง ทั้งด้านความเสถียร ความโปร่งใส และความน่าเชื่อถือของระบบ
จากข้อมูลของ DataReportal ล่าสุด พบว่า ผู้เล่นชาวไทยกว่า 63% ให้ความสำคัญกับบริการ คาสิโนสด และกว่า 45% ของกลุ่มนี้เชื่อมั่นว่า คาสิโนยูฟ่าเบท เป็นผู้ให้บริการที่มีมาตรฐานสูงทั้งในด้านเทคโนโลยี ความปลอดภัย และคุณภาพของระบบถ่ายทอดสด
คาสิโนสดคืออะไร?
คาสิโนสด (Live Casino) คือการเดิมพันผ่านระบบออนไลน์ที่เปิดโอกาสให้ผู้เล่นสามารถโต้ตอบกับ ดีลเลอร์จริง (Live Dealer) ได้แบบเรียลไทม์ ผ่านการถ่ายทอดจากสตูดิโอระดับนานาชาติ
ระบบนี้มอบบรรยากาศเหมือนอยู่ในคาสิโนจริง โดยยังคงความสะดวกจากการเล่นได้ทุกที่ ทุกเวลา ไม่ว่าจะเป็นบนมือถือหรือคอมพิวเตอร์
จุดเด่นของคาสิโนยูฟ่าเบท
ระบบ Live Streaming ของ UFABET รองรับความคมชัดระดับ Full HD
รองรับเสียงสองภาษา และอินเทอร์เฟซที่ใช้งานง่าย
สลับโต๊ะเดิมพันได้ทันทีโดยไม่ดีเลย์
ระบบมีความเสถียรสูง ทำงานต่อเนื่องได้อย่างลื่นไหลทั้งบนมือถือและคอมพิวเตอร์
คาสิโนยูฟ่าเบท รวมเกมถ่ายทอดสดยอดฮิตครบทุกประเภท

เกมไพ่ที่ได้รับความนิยมสูงในหมวดคาสิโนสด ด้วยรูปแบบการเล่นที่เข้าใจง่าย ใช้เวลาไม่นาน และมีโต๊ะหลายระดับให้เลือกตามงบประมาณของผู้เล่น
ระบบของ ยูฟ่าเบท (UFABET) ยังมีการแสดง สถิติย้อนหลัง แบบละเอียด ช่วยให้ผู้เล่นสามารถวิเคราะห์แนวโน้มและวางแผนการเดิมพันได้อย่างมีหลักการ

เสือมังกร (Dragon Tiger)
เกมไพ่ที่ขึ้นชื่อเรื่องความรวดเร็วและการตัดสินใจเฉียบคม ใช้ไพ่เพียงใบเดียวในการตัดสินผลแพ้–ชนะ ทำให้จบเกมไวและเข้าใจง่าย
เหมาะสำหรับผู้ที่ชอบเกมที่ดำเนินเร็วแต่ยังคงความตื่นเต้นในทุกจังหวะการเปิดไพ่

เกมวงล้อสุดคลาสสิกที่เปิดให้เดิมพันได้หลายรูปแบบ ไม่ว่าจะเป็นการเลือกตัวเลขเดี่ยว คู่–คี่ หรือสีแดง–ดำ
ระบบถ่ายทอดสดของ UFABET คาสิโนสด แสดงภาพการหมุนแบบ เรียลไทม์ (Real-Time) เพื่อความโปร่งใสและให้บรรยากาศใกล้เคียงกับการเล่นในคาสิโนจริง

ไฮโล (Sic Bo)
เกมลูกเต๋าที่ผู้เล่นชาวไทยคุ้นเคย ได้รับการพัฒนาให้ทันสมัยมากขึ้นด้วย กระดานเดิมพันแบบอินเทอร์แอคทีฟ
พร้อมระบบเสียงประกาศผลที่คมชัดทุกตา ทำให้การเล่นมีทั้งความสนุก ความชัดเจน และความมั่นใจในทุกการเดิมพัน
จุดเด่นของยูฟ่าคาสิโนสด ที่ทำให้แตกต่างจากเว็บทั่วไป
ภาพคมชัดระดับ Full HD
ระบบถ่ายทอดสดของ ยูฟ่าเบท (UFABET) ใช้เทคโนโลยีเดียวกับผู้ให้บริการสตรีมมิงระดับโลก ทำให้ภาพและเสียงมีความคมชัดสูง และมีเสถียรภาพตลอดการเล่น ผู้เล่นสามารถรับชมบรรยากาศของ คาสิโนสด ได้อย่างต่อเนื่องโดยไม่สะดุด หรือเกิดความหน่วงระหว่างเกม
ความโปร่งใสในทุกขั้นตอน
ทุกขั้นตอนของการเล่น ไม่ว่าจะเป็นการแจกไพ่ การหมุนวงล้อรูเล็ต หรือการเขย่าไฮโล ถ่ายทอดแบบ Real-Time โดยไม่มีการตัดต่อหรือแทรกสัญญาณ
ผู้เล่นสามารถเห็นทุกจังหวะการเล่นได้อย่างชัดเจนในแต่ละโต๊ะเดิมพัน
มั่นใจได้ว่าทุกเกมดำเนินไปด้วยความโปร่งใส ตรวจสอบได้จริง และยุติธรรมในทุกการวางเดิมพัน
ดีลเลอร์มืออาชีพ
ดีลเลอร์ของ ยูฟ่าคาสิโนสด ผ่านการคัดเลือกและฝึกอบรมตามมาตรฐานระดับสากล
เข้าใจรูปแบบการดำเนินเกมและการสื่อสารกับผู้เล่นอย่างมืออาชีพ
บรรยากาศภายในห้องเกมจึงเป็นกันเองและมีความสมจริง สร้างความรู้สึกเหมือนอยู่ในคาสิโนระดับพรีเมียม
รองรับการใช้งานทุกอุปกรณ์
ไม่ว่าจะใช้งานผ่าน iOS, Android, Windows หรือ macOS ผู้เล่นสามารถ เข้าสู่ระบบยูฟ่าเบท ได้ทันทีโดยไม่ต้องติดตั้งโปรแกรมเพิ่มเติม
ระบบได้รับการออกแบบให้ทำงานรวดเร็ว รองรับทั้งการเล่นผ่านมือถือและคอมพิวเตอร์ได้อย่างมีประสิทธิภาพ
ข้อแตกต่างระหว่าง คาสิโนสด กับ คาสิโนแบบเดิม ที่ผู้เล่นควรรู้
คาสิโนสดออนไลน์ มอบรูปแบบการเล่นที่โปร่งใสและน่าเชื่อถือมากกว่าระบบสุ่มแบบ RNG (Random Number Generator) เพราะทุกการเดิมพันเป็นการถ่ายทอดสดแบบ เรียลไทม์ (Real-Time) ที่ผู้เล่นสามารถมองเห็นขั้นตอนการดำเนินเกมได้ชัดเจน พร้อมพูดคุยหรือโต้ตอบกับ ดีลเลอร์จริง (Live Dealer) ได้โดยตรง
ซึ่งช่วยเพิ่มความสมจริงและทำให้บรรยากาศของเกมดูมีชีวิตชีวากว่าการเล่นผ่านระบบอัตโนมัติ
อีกทั้งระบบ คาสิโนสด ยังเปิดโอกาสให้ผู้เล่นพัฒนาทักษะด้านการวิเคราะห์ การอ่านเกม และการวางแผนได้ดีขึ้น ไม่ว่าจะเป็นผู้ที่เริ่มต้นเรียนรู้การเดิมพัน หรือผู้เล่นที่ต้องการต่อยอดสู่กลยุทธ์ขั้นสูง รูปแบบนี้ช่วยให้เข้าใจแนวทางของเกมได้อย่างเป็นระบบและมีประสิทธิภาพมากขึ้น
ด้วยองค์ประกอบเหล่านี้ คาสิโนสดยูฟ่าเบท (UFA BET Live Casino) จึงได้รับความนิยมเพิ่มขึ้นอย่างต่อเนื่อง ด้วยความโปร่งใส ความสมจริง และระบบที่ออกแบบมาเพื่อให้ผู้เล่นได้รับประสบการณ์ที่ครบถ้วนกว่าคาสิโนแบบดั้งเดิม
ตารางเปรียบเทียบ ค่ายคาสิโนออนไลน์ยูฟ่าเบท
| รายการเปรียบเทียบ | Sexy Gaming | SA Gaming | Evolution Gaming |
|---|---|---|---|
| จุดเด่น | ดีลเลอร์สาวสวย สร้างบรรยากาศสนุกและเป็นกันเอง | ระบบเสถียรสูง มีข้อมูลสถิติย้อนหลังให้ดูอย่างละเอียด | ระดับสากล เกมครบทุกแนว ห้องเล่นหลายแบบ |
| ประเภทเกมหลัก | บาคาร่า, เสือมังกร, รูเล็ต, ไฮโล | บาคาร่า, เสือมังกร, รูเล็ต, ไฮโล | Baccarat, Lightning Roulette, Game Show |
| คุณภาพการถ่ายทอดสด | ภาพชัดระดับ Full HD พร้อมเสียงบรรยายภาษาไทย | ระบบชัดและนิ่ง รองรับการเล่นผ่านมือถือได้ดี | คุณภาพระดับ Ultra HD พร้อมมุมกล้องหลายจุดและเสียงภาษาอังกฤษ |
| จุดเด่นเฉพาะของระบบ | เล่นได้หลายโต๊ะในเวลาเดียวกัน ดีลเลอร์เต้นสร้างสีสันระหว่างเกม | โหมดซูเปอร์ซิก และระบบอ่านเค้าไพ่แบบละเอียด | มีเกมโชว์สด โบนัสสุ่ม และระบบ Multi-Camera เพิ่มความตื่นเต้น |
| เหมาะกับผู้เล่นประเภทใด | เหมาะกับคนที่ชอบความสนุกสนานและเล่นแบบผ่อนคลาย | เหมาะกับผู้ที่เน้นวิเคราะห์เกมและดูสถิติประกอบ | เหมาะกับผู้เล่นที่ต้องการความแปลกใหม่และความหลากหลายของเกม |
| ภาษาที่รองรับ | ไทย, อังกฤษ | ไทย, อังกฤษ | อังกฤษ, จีน, ญี่ปุ่น, เยอรมัน |
Slot Online (เกมสล็อต)
ยูฟ่าเบท สล็อตแตกง่าย เล่นได้ทุกวัน รวมเกมสล็อตออนไลน์กว่า 1,000 เกม
ในหมวด เกมคาสิโนออนไลน์ หนึ่งในประเภทที่ได้รับความนิยมอย่างต่อเนื่องคือ สล็อตออนไลน์ (Online Slots) เพราะเป็นเกมที่มีกติกาเข้าใจง่าย ใช้เวลาไม่นาน และไม่จำเป็นต้องมีเทคนิคซับซ้อน แต่สามารถสร้างความเพลิดเพลินและผลตอบแทนได้จริง โดยเฉพาะบนระบบของ สล็อตยูฟ่าเบท (UFABET Slots) ซึ่งได้รับการยอมรับว่าเป็นหนึ่งในเว็บที่รวมเกมสล็อต แตกง่ายที่สุด และมีจำนวนเกมให้เลือกเล่นมากที่สุดในภูมิภาคเอเชีย
จากรายงานของ Slot Industry Report ล่าสุด ระบุว่า ยูฟ่าเบท (UFABET) มีเกมสล็อตจากค่ายชั้นนำมากกว่า 1,000 เกม พร้อมอัตราการจ่ายโบนัสเฉลี่ย (Bonus Payout Rate) อยู่ระหว่าง 94% – 97% ซึ่งสูงกว่าค่าเฉลี่ยของตลาดโลก (Global RTP) อย่างชัดเจน
สล็อตแตกง่ายคืออะไร?
คำว่า “สล็อตแตกง่าย (Easy Win Slot)” หมายถึงเกมสล็อตที่ถูกออกแบบให้มีอัตราการจ่ายรางวัลหรือโบนัสออกถี่กว่าปกติ โดยภายในเกมจะมีระบบ ฟรีสปิน (Free Spin) และ แจ็กพอต (Jackpot) ซึ่งเพิ่มมูลค่ารางวัลให้สูงขึ้นหลายเท่าในระยะเวลาสั้น ๆ ด้วยรูปแบบการเล่นที่เข้าใจง่ายและมีจังหวะลุ้นอยู่ตลอดเวลา จึงเป็นเหตุผลที่ทำให้ผู้เล่นจำนวนมากเลือกเล่นสล็อตกับ UFABET เป็นประจำ
ยูฟ่าเบท รวมค่ายสล็อตแตกง่าย ครบทั้งเกมใหม่และคลาสสิก

หรือที่รู้จักกันในชื่อ พีจีสล็อต (PG Slot) โดดเด่นด้วยกราฟิกที่คมชัด สีสันสดใส และธีมเกมที่มีเอกลักษณ์ เช่น Fortune Mouse, Mahjong Ways และ Leprechaun Riches
ทุกเกมถูกพัฒนาโดยทีมงานมืออาชีพระดับสากล เน้นความเสถียรของระบบและอัตราการจ่ายที่ยุติธรรม

ค่ายสล็อตยอดนิยมที่ขึ้นชื่อเรื่อง แจ็กพอตแตกง่าย และระบบเกมที่เข้าใจง่าย
เกมเด่นอย่าง Roma และ Chilli Hunter ได้รับความนิยมอย่างกว้างขวางในหมู่นักเล่นสล็อตทั่วเอเชีย
ด้วยรูปแบบการเล่นที่ไม่ซับซ้อนแต่ให้ผลตอบแทนคุ้มค่า

ค่ายเกมที่มุ่งเน้นความรวดเร็วและความตื่นเต้นในการเล่น
มีจุดเด่นคือ ฟรีสปินออกบ่อย และระบบ ซื้อโบนัส (Feature Buy) ที่ช่วยให้ผู้เล่นสามารถลุ้นรางวัลได้ทันที
เกมยอดนิยมอย่าง Sweet Bonanza และ The Dog House ยังคงเป็นที่ชื่นชอบในระดับสากลอย่างต่อเนื่อง

UFASLOT
ค่ายเกมภายในเครือ ยูฟ่าเบท (UFABET) ที่รวบรวมเกมสล็อตทั้งแนวคลาสสิกและแนวทันสมัยไว้ครบในระบบเดียว
รองรับผู้เล่นทุกกลุ่ม ไม่ว่าจะชอบสไตล์โบนัสแตกง่ายหรือเน้นกราฟิกคุณภาพสูง
ระบบของ UFASLOT ถูกออกแบบให้ใช้งานง่าย เสถียร และรองรับการเล่นได้บนทุกอุปกรณ์
ตารางเปรียบเทียบค่าย เกมสล็อตออนไลน์ ยูฟ่าเบท
| รายการเปรียบเทียบ | PG Slot | Joker Gaming | Pragmatic Play |
|---|---|---|---|
| ค่าเฉลี่ย RTP (Return to Player) | 96.5% – 97.2% | 95.0% – 96.5% | 96.5% – 97.0% |
| อัตราการแตกของโบนัส (Bonus Rate) | โบนัสออกถี่ โดยเฉพาะเกมยอดนิยมอย่าง Lucky Neko | โบนัสแตกแรงในบางเกม เช่น Roma | แตกต่อเนื่อง เสถียร มีระบบซื้อรอบโบนัสจำนวนมาก |
| จุดเด่นของระบบเกม | ภาพสวย เคลื่อนไหวสมจริง ฟรีสปินออกสุ่มบ่อย | เล่นง่าย รอบเกมสั้น มีระบบคอมโบเพิ่มรางวัล | ซื้อฟรีสปินได้ (Feature Buy) และบางเกมมี Super Bonus |
| เหมาะกับผู้เล่นประเภทใด | เหมาะกับผู้ที่ชอบกราฟิกสวย เล่นง่าย เข้าใจระบบไว | เหมาะกับคนที่ชอบเกมหมุนเร็ว เล่นได้หลายรอบต่อบิล | เหมาะกับสายวางแผน ทำกำไรจากโบนัส และชอบระบบเสริมในเกม |
| รองรับการใช้งานบนมือถือ | ✅ รองรับทั้ง iOS และ Android | ✅ รองรับทั้ง iOS และ Android | ✅ รองรับทั้ง iOS และ Android |
| เกมยอดนิยม | Lucky Neko, Mahjong Ways, Treasures of Aztec | Roma, Chilli Hunter, Black Beard Legacy | Sweet Bonanza, Gates of Olympus, The Dog House |
R1UFABET (Special Bet)
R1UFABET บริการเดิมพันกีฬาเฉพาะ ภายใต้มาตรฐาน ยูฟ่าเบท
หนึ่งในกลยุทธ์หลักที่ช่วยให้เครือ UFABET ขยายฐานผู้เล่นได้อย่างต่อเนื่อง คือการเปิดบริการ R1UFABET (อาร์วันยูฟ่าเบท) ซึ่งถูกพัฒนาเพื่อรองรับผู้ที่ชื่นชอบการเดิมพันใน กีฬาเฉพาะทาง ไม่ว่าจะเป็น แทงมวยออนไลน์, ไก่ชนออนไลน์, วัวชน, หรือ แทงวอลเลย์บอลสด โดยระบบนี้ออกแบบมาเพื่อให้ตอบสนองต่อผู้เล่นที่ต้องการรูปแบบการเดิมพันเฉพาะทางอย่างแท้จริง
จากรายงานของ Digital Behavior Lab พบว่า ผู้ใช้งานชาวไทยกว่า 28% ให้ความสนใจกีฬาที่ไม่ใช่ฟุตบอล และต้องการเว็บไซต์ที่มีระบบเฉพาะทางในการเล่นและรับชมการแข่งขัน จึงเป็นที่มาของการพัฒนา R1UFABET ให้เป็นบริการที่แยกออกมาจากระบบหลักของยูฟ่าเบท เพื่อรองรับกลุ่มผู้เล่นที่เน้นการเดิมพันกีฬาท้องถิ่นและกีฬาพื้นบ้านโดยเฉพาะ
R1UFABET คืออะไร?
R1UFABET คือบริการเสริมจากระบบหลักของ ยูฟ่าเบท (UFABET) ซึ่งใช้โครงสร้างความปลอดภัยเดียวกัน (Unified Security Infrastructure) แต่เน้นให้บริการด้าน กีฬาพื้นบ้านและกีฬาเฉพาะกลุ่ม เช่น มวยไทย, วัวชน, ไก่ชน และ วอลเลย์บอล โดยมีระบบถ่ายทอดสดและโครงสร้างการเดิมพันที่เข้าใจง่าย ครอบคลุมทุกรูปแบบการเล่น เพื่อให้ผู้ใช้ได้รับประสบการณ์การเล่นที่ครบถ้วนและเหมาะกับกีฬาที่ตนสนใจมากที่สุด
รวมกีฬาพื้นบ้านและกีฬาเฉพาะทาง ที่เดิมพันได้เฉพาะใน R1UFA

ระบบเดิมพันมวยของ R1UFA ถ่ายทอดสดจากเวทีมวยชื่อดังทั่วประเทศ เช่น ราชดำเนิน, ลุมพินี และสนามระดับภูมิภาค
ผู้เล่นสามารถเลือกวางเดิมพันได้ทั้งแบบ ยกต่อยก หรือ เต็มเวลา โดยราคาต่อรองมีการอัปเดตตามสถานการณ์จริงของการแข่งขัน
เหมาะสำหรับผู้ที่ติดตามศึก มวยไทย R1 และผู้ที่ชื่นชอบการเดิมพันเชิงวิเคราะห์ในรูปแบบกีฬาไทยแท้

หนึ่งในกีฬาเฉพาะทางที่ได้รับความนิยมอย่างต่อเนื่อง โดยระบบของ R1UFA ถ่ายทอดสดตรงจากสนามจริงในพื้นที่ภาคใต้
มาพร้อมข้อมูลสถิติของวัวแต่ละตัวก่อนการแข่งขัน และอัปเดตราคาต่อรองแบบ Real-Time
ครอบคลุมทั้งรายการ วัวชน R1 และการแข่งขันจากสนามท้องถิ่น ภายใต้ระบบมาตรฐานเดียวกับ UFA

สำหรับผู้ที่ติดตามการแข่งขัน ไก่ชน R1 หรือบ่อนไก่ชื่อดังจากทั่วประเทศ
ระบบของ R1UFA ถ่ายทอดสดทุกคู่ พร้อมฟังก์ชัน รีเพลย์ และ ไฮไลต์ย้อนหลัง
รวมถึงราคาต่อรองที่เปลี่ยนแปลงตามรูปเกมแบบเรียลไทม์
ช่วยให้ผู้เล่นสามารถวางเดิมพันได้อย่างมั่นใจ ด้วยระบบที่โปร่งใสและตรวจสอบได้ทุกขั้นตอน
R1BET เปิดให้เดิมพันวอลเลย์บอลครบทุกระดับ ทั้งลีกต่างประเทศและรายการระดับทีมชาติ
มีระบบ Live Score, สถิติย้อนหลัง, และ ราคาต่อรองอัตโนมัติ ที่อัปเดตตลอดการแข่งขัน
เหมาะสำหรับผู้เล่นที่ติดตามการแข่งขันแบบ วอลเลย์บอลสด และต้องการวิเคราะห์เกมแบบเรียลไทม์เพื่อเพิ่มความแม่นยำในการวางเดิมพัน
R1BET มั่นใจทุกการใช้งาน ด้วยระบบอัปเดตและธุรกรรมอัตโนมัติ
อัปเดตราคาทันที (Real-Time Update)
ระบบของ R1BET ถูกออกแบบให้ข้อมูลราคาต่อรองและผลการแข่งขันอัปเดตแบบเรียลไทม์
ผู้เล่นสามารถวิเคราะห์และวางเดิมพันได้อย่างแม่นยำตลอดการแข่งขันความมั่นคงทางการเงินในมาตรฐานยูฟ่าเบท
R1BET ใช้ระบบธุรกรรมเดียวกับ เว็บพนันออนไลน์ยูฟ่าเบท (UFABET)
รองรับทั้งบัญชีธนาคารภายในประเทศและ QR Code Payment
ทุกการฝาก–ถอนทำงานอัตโนมัติภายในไม่เกินหนึ่งนาที ช่วยให้ผู้เล่นมั่นใจได้ในความรวดเร็วและปลอดภัยของทุกธุรกรรมอินเทอร์เฟซใช้งานง่าย รองรับสองภาษา
หน้าเว็บและระบบเดิมพันของ R1BET ถูกออกแบบให้ใช้งานสะดวกทั้งบนมือถือและคอมพิวเตอร์
รองรับภาษาไทยและภาษาอังกฤษอย่างครบถ้วน เพื่อให้ใช้งานได้ง่ายสำหรับผู้เล่นทุกกลุ่มระบบป้องกันข้อมูลขั้นสูง (Data Encryption)
ข้อมูลสมาชิกทั้งหมดถูกปกป้องด้วยระบบเข้ารหัสตามมาตรฐานสากล
เพื่อรักษาความปลอดภัยและความเป็นส่วนตัวของผู้เล่น ป้องกันการเข้าถึงข้อมูลจากบุคคลภายนอกอย่างมีประสิทธิภาพ
ความน่าเชื่อถือของ R1UFA ที่เติบโตภายใต้มาตรฐาน เว็บยูฟ่า
ด้วยการเป็นส่วนหนึ่งของเครือข่ายหลัก ยูฟ่า เบท ทำให้ R1 UFA BET ได้รับการยอมรับอย่างรวดเร็วในกลุ่มผู้เล่นชาวไทย โดยเฉพาะผู้ที่ชื่นชอบกีฬาพื้นบ้านและกีฬาเฉพาะทาง เช่น มวยไทย, วัวชน, และ ไก่ชน ซึ่งเป็นกลุ่มที่ให้ความสำคัญกับความโปร่งใส ความยุติธรรม และความถูกต้องของระบบเดิมพัน
R1UFA ใช้มาตรฐานเดียวกับเว็บเดิมพันระดับโลก ทั้งในด้านเสถียรภาพของระบบ ความรวดเร็วในการทำธุรกรรม และความแม่นยำของการตรวจสอบผล ผู้เล่นจึงสามารถมั่นใจได้ว่าทุกการเดิมพันดำเนินไปอย่างปลอดภัยและตรวจสอบได้จริง
นอกจากนี้ R1UFA ยังมีทีม Support Professional ให้บริการตลอด 24 ชั่วโมง ผ่านช่องทาง Live Chat, LINE Official, และ ระบบ Support Ticket เพื่อให้ผู้เล่นได้รับความช่วยเหลืออย่างรวดเร็ว ทั้งในด้านเทคนิค การใช้งาน และข้อมูลที่เกี่ยวข้องกับการเดิมพัน
ยูฟ่าเบทพัฒนาระบบออโต้ ฝากถอนง่าย รองรับทุกธนาคาร และ QR Payment
ความรวดเร็วและความสะดวกคือหัวใจสำคัญของบริการยุคดิจิทัล ยูฟ่า เบท หรือ UFA จึงพัฒนา ระบบฝาก–ถอนอัตโนมัติ (Auto Deposit & Auto Withdrawal) ให้ผู้เล่นทำรายการได้เองโดยไม่ต้องแจ้งสลิปหรือผ่านแอดมิน
ระบบรองรับ ทุกธนาคารในไทย และ QR Code Payment ทำให้ฝาก–ถอนเสร็จภายในไม่กี่วินาที เพิ่มความคล่องตัวและความมั่นใจในการใช้งาน
ตามข้อมูลจาก eGambling Tech Lab เว็บไซต์ที่ใช้ระบบออโต้มีอัตราการใช้งานซ้ำเพิ่มกว่า 38% ภายในสามเดือน แสดงถึงประสิทธิภาพของระบบที่ช่วยลดขั้นตอนและข้อผิดพลาด
ทำให้การทำธุรกรรมรวดเร็วและราบรื่นกว่าระบบเดิมอย่างชัดเจน
ระบบฝาก–ถอนออโต้ UFA BET คืออะไร?
ระบบฝาก–ถอนออโต้ของ เว็บยูฟ่า คือบริการที่เปิดให้ผู้เล่นสามารถทำรายการได้ด้วยตัวเอง โดยไม่ต้องติดต่อเจ้าหน้าที่หรืออัปโหลดสลิปโอนเงิน เพียงกรอกยอดที่ต้องการ ระบบจะตรวจสอบและปรับเครดิตให้อัตโนมัติภายในเวลาไม่เกิน 30 วินาที ทุกขั้นตอนดำเนินการด้วยความรวดเร็ว โปร่งใส และปลอดภัย
รองรับธนาคารอะไรบ้าง?
ระบบอัตโนมัติของ ยูฟ่าเบท รองรับการทำธุรกรรมกับธนาคารชั้นนำในประเทศ ได้แก่
กสิกรไทย (KBank)
ไทยพาณิชย์ (SCB)
กรุงไทย (KTB)
กรุงศรีอยุธยา (BAY)
ทีเอ็มบีธนชาต (TTB)
นอกจากนี้ ยังรองรับการโอนผ่าน Mobile Banking ทุกค่าย
รวมถึงการทำรายการผ่านระบบ QR Payment เพื่อความสะดวกสูงสุดของผู้ใช้งาน
วิธีใช้งานระบบฝาก–ถอนออโต้ ยูฟ่าเบท


เข้าสู่ระบบสมาชิก UFABET
สามารถล็อกอินได้ทั้งบนมือถือและคอมพิวเตอร์ รองรับทุกระบบปฏิบัติการทั้ง iOS, Android, และ Windowsเลือกเมนู “ฝากเงิน” หรือ “ถอนเงิน”
จากหน้าแดชบอร์ดเลือกช่องทางที่ต้องการทำธุรกรรม เช่นMobile Banking
QR Code Payment
TrueMoney Wallet
ระบุยอดที่ต้องการทำรายการ
ระบบจะสร้างหมายเลขบัญชีเฉพาะ หรือ QR Code สำหรับการฝากเงินแบบอัตโนมัติระบบปรับเครดิตอัตโนมัติ
หลังจากโอนเงิน ระบบจะตรวจสอบและปรับยอดให้ทันทีภายในไม่กี่วินาที
ไม่ต้องส่งสลิปหรือรอเจ้าหน้าที่ตรวจสอบขั้นตอนการถอนเงิน
เลือก “ถอนเงิน” จากเมนูหลัก กรอกจำนวนเงินและบัญชีปลายทาง
ระบบจะโอนเข้าบัญชีโดยอัตโนมัติภายในประมาณ 1–3 นาที
ระบบทั้งหมดของ UFA Auto ได้รับการออกแบบให้เชื่อมต่อกับธนาคารชั้นนำของไทยโดยตรง เพื่อให้ทุกขั้นตอนของการฝาก–ถอนมีความรวดเร็ว ปลอดภัย และโปร่งใสในระดับมาตรฐานเดียวกับสถาบันการเงินสากล




ระบบออโต้เว็บยูฟ่า ฝากถอนรวดเร็ว มั่นใจทุกธุรกรรม
ฝาก–ถอนรวดเร็วแบบ Real-Time
ระบบของ ยูฟ่าเบท (UFABET) ประมวลผลธุรกรรมทันทีหลังทำรายการ
ใช้เวลาเพียงไม่กี่วินาทีในการอัปเดตยอดเครดิตแบบอัตโนมัติรองรับทุกธนาคารและกระเป๋าเงินดิจิทัลในไทย
รองรับการทำรายการผ่าน กสิกรไทย, ไทยพาณิชย์, กรุงไทย,
รวมถึงกระเป๋าเงินอิเล็กทรอนิกส์ (E-Wallet) เพื่อเพิ่มความสะดวกสูงสุดให้กับผู้ใช้งานตรวจสอบรายการอัตโนมัติ ตลอด 24 ชั่วโมง
ผู้เล่นสามารถทำธุรกรรมได้ทุกเวลา โดยระบบจะตรวจจับและยืนยันผลอัตโนมัติ
ลดขั้นตอนการรออนุมัติจากเจ้าหน้าที่ให้หมดไประบบความปลอดภัยระดับสถาบันการเงิน
ใช้เทคโนโลยีการเข้ารหัสแบบ AES-256 Bit และระบบ Tokenization
เหมือนกับที่บริษัทด้านการเงินระดับโลกใช้ เพื่อป้องกันข้อมูลและธุรกรรมของผู้เล่นอย่างครบถ้วน
เว็บพนันยูฟ่าเบท ระบบการเงินรวดเร็ว เสถียร แม่นยำ
จากสถิติย้อนหลัง 6 เดือน พบว่า เว็บพนันยูฟ่าเบท มีความเร็วในการทำธุรกรรมที่โดดเด่นกว่าเว็บทั่วไปอย่างเห็นได้ชัด โดยใช้เวลาเฉลี่ยในการ ฝากเงินเพียง 19 วินาที และ ถอนเงินเฉลี่ย 2 นาที 15 วินาที
เมื่อเปรียบเทียบกับเว็บทั่วไปที่ใช้เวลาระหว่าง 5–10 นาทีต่อรายการ โดยเฉพาะช่วงที่มีผู้เล่นหนาแน่น ระบบของ เว็บ UFA ยังคงเสถียรและตรวจสอบได้แบบอัตโนมัติ ซึ่งถือเป็นอีกหนึ่งจุดแข็งสำคัญที่ช่วยให้การใช้งานราบรื่น และสร้างความเชื่อมั่นให้กับผู้เล่นในทุกขั้นตอนของการทำธุรกรรม
สมัคร UFABET ง่ายเพียง 3 ขั้นตอน พร้อมสิทธิพิเศษสำหรับผู้เล่นใหม่
หนึ่งในเหตุผลที่ทำให้ เว็บยูฟ่าเบทออนไลน์ ได้รับความนิยมสูงอย่างต่อเนื่อง คือขั้นตอนการสมัครสมาชิกที่เรียบง่ายและใช้เวลาไม่นาน ผู้เล่นสามารถเริ่มใช้งานได้ทันทีภายในไม่กี่นาที โดยไม่ต้องยื่นเอกสารเพิ่มเติม ระบบที่ออกแบบให้ใช้งานสะดวกนี้เอง ทำให้คำว่า “สมัครยูฟ่าเบท” กลายเป็นหนึ่งในคำค้นที่ถูกพูดถึงมากทั้งในประเทศไทยและต่างประเทศ
จากข้อมูลของ eGaming Insight พบว่า เว็บไซต์ที่มีขั้นตอนสมัครไม่เกิน 3 คลิก มีอัตราการสมัครสำเร็จสูงกว่าค่าเฉลี่ยทั่วไปถึง 41% ซึ่งสอดคล้องกับแนวทางของ พนัน UFA ที่พัฒนาระบบให้รองรับพฤติกรรมผู้เล่นยุคใหม่ที่ต้องการความรวดเร็วและความชัดเจนในทุกขั้นตอน
สิ่งที่ต้องใช้ในการ สมัคร UFABET
การสมัครสมาชิกกับ ยูฟ่าเบท ใช้ข้อมูลเพียงไม่กี่อย่าง ได้แก่
หมายเลขโทรศัพท์ที่สามารถรับ รหัสยืนยัน OTP ได้
บัญชีธนาคารที่ชื่อเจ้าของตรงกับผู้สมัคร เช่น กสิกรไทย, ไทยพาณิชย์, หรือ กรุงไทย
ผู้สมัครต้องมีอายุ 18 ปีขึ้นไป
ไม่จำเป็นต้องแนบเอกสารเพิ่มเติม สามารถเริ่มใช้งานได้ทันทีผ่านหน้าเว็บไซต์หลักของ UFA
ความปลอดภัยในการสมัครสมาชิก
ระบบ UFABET Member Login ใช้มาตรฐานการรักษาความปลอดภัยแบบ AES-256 Encryption เทียบเท่ากับระบบของสถาบันการเงินระดับโลก พร้อมระบบยืนยันตัวตนด้วย OTP (One-Time Password) เพื่อป้องกันการเข้าถึงข้อมูลจากบุคคลภายนอก ผู้เล่นจึงมั่นใจได้ว่าข้อมูลส่วนตัวและธุรกรรมทั้งหมด อยู่ภายใต้การปกป้องในระดับสูงสุดตลอดการใช้งาน
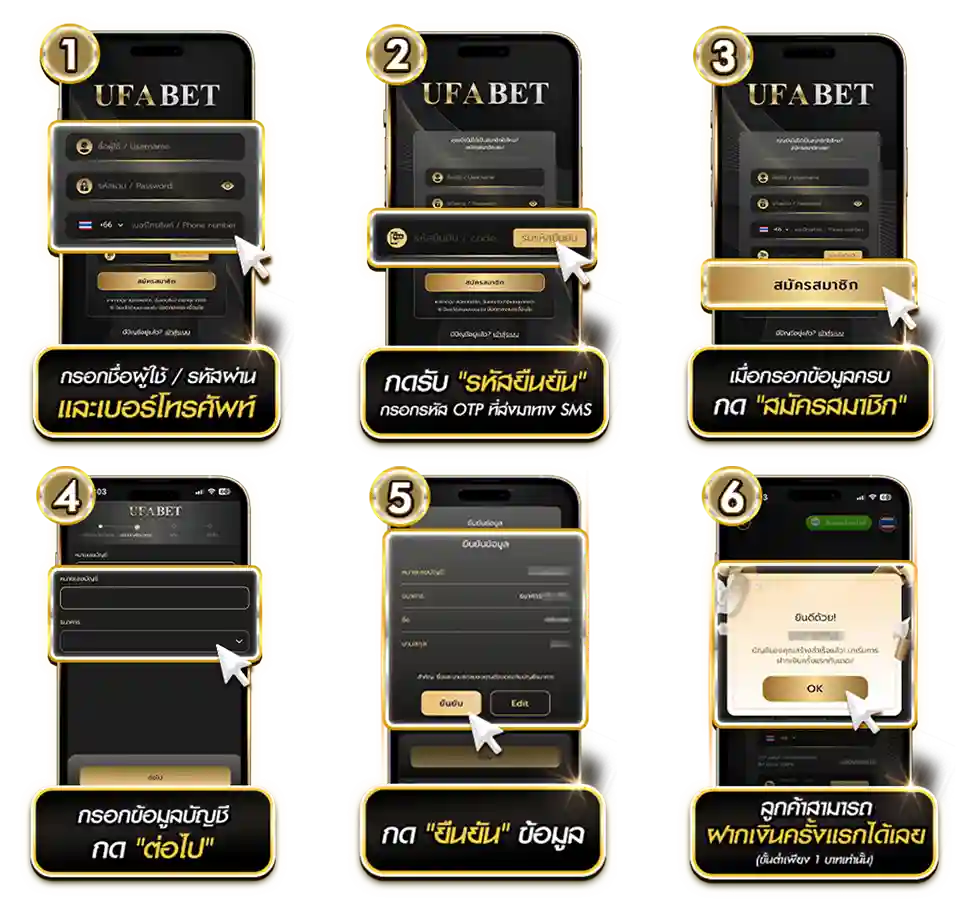
วิธีสมัครยูฟ่าเบท 3 ขั้นตอนง่าย ๆ เริ่มเล่นได้ทันที
กรอกข้อมูลเบื้องต้น
ไปที่หน้าเว็บไซต์หลักของ ยูฟ่าเบท แล้วเลือก “สมัครสมาชิก”
จากนั้นกรอกข้อมูลพื้นฐาน ได้แก่ หมายเลขโทรศัพท์ บัญชีธนาคารที่ตรงกับชื่อจริง และตั้งรหัสผ่านเพื่อใช้ในการเข้าสู่ระบบยืนยันตัวตนผ่านรหัส OTP
ระบบจะส่ง รหัสยืนยัน (OTP) ไปยังหมายเลขโทรศัพท์ที่ลงทะเบียนไว้
เพียงกรอกรหัสที่ได้รับเพื่อยืนยันความถูกต้องของข้อมูล ระบบจะทำการตรวจสอบทันทีและดำเนินการสมัครให้เสร็จสมบูรณ์อย่างปลอดภัยเข้าสู่ระบบอัตโนมัติ
เมื่อยืนยันข้อมูลเรียบร้อยแล้ว ระบบจะพาผู้ใช้เข้าสู่บัญชีโดยอัตโนมัติ
จากนั้นสามารถเติมเครดิตและเริ่มเดิมพันได้ทันที ทั้ง บาคาร่า, สล็อตออนไลน์, และ แทงบอลเว็บตรง ยูฟ่าเบท

วิธีแทงบอลยูฟ่าเบทบนมือถือ

วิธีแทงมวย R1BET บนมือถือ
ยูฟ่าเบทบนมือถือ ครบทุกระบบ รองรับทั้ง iOS Android และ PC
ยูฟ่าเบทมือถือ ได้กลายเป็นส่วนสำคัญของระบบเดิมพันยุคปัจจุบัน โดย ยูฟ่าเบท คือหนึ่งในเว็บไซต์ที่พัฒนาระบบด้วยแนวคิด Mobile-First Design มุ่งเน้นความเร็ว ความเสถียร และการใช้งานที่สะดวกในทุกอุปกรณ์ รองรับทั้ง iOS, Android และ PC โดยไม่จำเป็นต้องดาวน์โหลดหรือติดตั้งแอปเพิ่มเติม
ข้อมูลจาก Global Betting UX Report ระบุว่า เว็บไซต์ที่โหลดหน้าแรกได้ภายใน 3 วินาที มีอัตราการใช้งานต่อเนื่องเพิ่มขึ้นกว่า 53% และ Bounce Rate ลดลงมากกว่า 38% ซึ่งสอดคล้องกับแนวทางของ เว็บ UFA ที่พัฒนาอินเทอร์เฟซให้ตอบรับการใช้งานผ่านมือถือได้อย่างเต็มประสิทธิภาพ
เว็บยูฟ่าเบทบนมือถือใช้งานได้หรือไม่?
สามารถใช้งานได้ครบทุกฟังก์ชันเต็มรูปแบบ ผู้เล่นสามารถเข้าสู่ระบบได้ทันทีผ่านเบราว์เซอร์ เช่น Chrome หรือ Safari
โดยไม่ต้องติดตั้งแอปพลิเคชัน ไม่ว่าจะเป็นการ แทงบอล, เล่นคาสิโนสด, สล็อตยูฟ่า, หรือ หวยออนไลน์
ทุกบริการทำงานได้อย่างลื่นไหลเทียบเท่ากับการใช้งานบนคอมพิวเตอร์รองรับระบบใดบ้าง?
เว็บไซต์ของ ยูฟ่าเบท รองรับทุกระบบปฏิบัติการหลัก ทั้ง iOS, Android, Windows และ MacOS
สามารถใช้งานได้ทั้งบนมือถือ แท็บเล็ต และคอมพิวเตอร์ โดยระบบถูกออกแบบให้ปรับขนาดหน้าจออัตโนมัติเพื่อความสะดวกสูงสุดระบบมีความเสถียรแค่ไหน?
UFA ใช้เทคโนโลยี Web Progressive App (PWA) ร่วมกับ Content Delivery Network (CDN) ระดับสากล
ช่วยให้เว็บไซต์โหลดหน้าได้รวดเร็วและคงความเสถียรแม้ในพื้นที่ที่สัญญาณอินเทอร์เน็ตไม่แรง
จึงสามารถใช้งานได้อย่างต่อเนื่องโดยไม่สะดุดในทุกช่วงเวลา
ยูฟ่าเบทบนมือถือ ใช้งานง่ายไม่ต้องโหลดแอป รองรับทุกระบบ
ไม่ต้องติดตั้งแอปพลิเคชัน
ผู้เล่นสามารถใช้งานได้ทันทีผ่านเว็บเบราว์เซอร์ เช่น Chrome, Safari, หรือ Edge
โดยไม่ต้องดาวน์โหลดหรือติดตั้งแอปเพิ่มเติม ช่วยให้เข้าเล่นได้สะดวกและไม่ต้องคอยอัปเดตเวอร์ชันอินเทอร์เฟซออกแบบตามหลัก User-Centered Design
โครงสร้างเมนูและปุ่มต่าง ๆ ถูกจัดเรียงให้เหมาะกับการใช้งานบนหน้าจอมือถือ
รองรับทั้งแนวตั้งและแนวนอน เพื่อให้ผู้เล่นใช้งานได้ราบรื่นในทุกหมวด เช่น คาสิโนสด ยูฟ่าเบท, สล็อตออนไลน์, และ แทงบอล ยูฟ่าเบทระบบป้องกันการหลุดระหว่างใช้งาน
โดยเฉพาะในโหมด คาสิโนสดบนมือถือ, หากสัญญาณอินเทอร์เน็ตขาดหรือสะดุด
ระบบจะทำการเชื่อมต่อใหม่ (Reconnect) โดยอัตโนมัติ เพื่อให้ผู้เล่นกลับเข้าสู่เกมได้ทันทีโดยไม่เสียสิทธิ์ในการเดิมพัน
เว็บ UFA พัฒนาระบบให้ทำงานเร็ว เสถียร และประหยัดดาต้า
ระบบของ UFA เว็บพนันออนไลน์ ถูกออกแบบมาให้ตอบสนองต่อผู้ใช้งานได้รวดเร็วและมีเสถียรภาพสูง ด้วยเทคโนโลยี Page Preloading และระบบปรับภาพ–คอนเทนต์อัตโนมัติ (Adaptive Rendering) ทำให้หน้าเว็บไซต์โหลดเฉลี่ยภายในเวลาเพียง 1.5 – 2.3 วินาที ทั้งบนเครือข่าย 4G และ Wi-Fi ซึ่งเร็วกว่าค่าเฉลี่ยของเว็บเดิมพันทั่วไปอย่างเห็นได้ชัด
นอกจากนี้ ยังมีระบบ Cache Management ที่ช่วยให้หน้าเกมที่เคยเข้าเล่นโหลดซ้ำได้เร็วขึ้น พร้อมลดการใช้ดาต้าอินเทอร์เน็ต เหมาะสำหรับผู้ที่ใช้งานบนมือถือเป็นประจำ จึงทำให้ เว็บ UFA เป็นหนึ่งในระบบที่ให้ความสำคัญกับความเร็ว ความเสถียร และการใช้งานอย่างต่อเนื่องในทุกสถานการณ์
ระบบเกมยูฟ่าเบท เสถียร ปลอดภัย เล่นได้ต่อเนื่องทุกอุปกรณ์

แทงบอลออนไลน์
ระบบเดิมพันฟุตบอลของ ยูฟ่าเบท ได้รับการพัฒนาให้ราคาบอลและค่าน้ำอัปเดตแบบ เรียลไทม์ (Real-Time Odds Update)
โดยไม่ต้องรีเฟรชหน้าเว็บไซต์ ผู้เล่นสามารถติดตามการเปลี่ยนแปลงของราคาได้ทันทีระหว่างการแข่งขัน
ช่วยให้วางเดิมพันได้อย่างแม่นยำและต่อเนื่องตลอดเกม

คาสิโนสดออนไลน์ UFA
การถ่ายทอดสดในหมวดคาสิโนของ UFA ใช้มาตรฐานภาพระดับ Full HD พร้อมเทคโนโลยี Adaptive Bitrate Streaming ที่ปรับคุณภาพของภาพและเสียงให้เหมาะสมกับความเร็วอินเทอร์เน็ตโดยอัตโนมัติ ผู้เล่นจึงสามารถเพลิดเพลินกับเกมได้อย่างต่อเนื่อง ภาพและเสียงคมชัด ไม่มีสะดุด

สล็อตแตกง่าย บนมือถือ
เกมสล็อตจาก ยูฟ่าเบท ผ่านการปรับระบบให้ทำงานได้อย่างเสถียรบนทุกอุปกรณ์
ทั้งมือถือ แท็บเล็ต และคอมพิวเตอร์ ผู้เล่นสามารถเข้าเล่นได้อย่างต่อเนื่องโดยไม่หลุดจากระบบ
พร้อมกราฟิกที่คมชัดและเอฟเฟกต์ที่ทำงานลื่นไหลในทุกเวอร์ชันการใช้งาน
มาตรการความปลอดภัยยูฟ่าเบท ควบคู่การดูแลผู้เล่นตลอด 24 ชม.
ความปลอดภัยของระบบถือเป็นหัวใจหลักที่ผู้เล่นให้ความสำคัญในการใช้งานเว็บเดิมพันออนไลน์ โดยเฉพาะในส่วนของ ข้อมูลส่วนบุคคล และ ธุรกรรมทางการเงิน ซึ่ง เว็บเดิมพันยูฟ่าเบท ได้รับการยอมรับอย่างต่อเนื่องว่าเป็นหนึ่งในเว็บไซต์ที่ให้ความสำคัญกับมาตรการป้องกันระดับสูง ทั้งในเชิงเทคนิคและกระบวนการดูแลผู้ใช้งาน สามารถศึกษารายละเอียดเพิ่มเติมได้ใน คู่มือการใช้งาน UFABET
จากรายงานของ WebTrust Security Index ล่าสุด ยูฟ่าเบทได้รับคะแนนด้าน “ความปลอดภัยของข้อมูลผู้ใช้” และ “ความโปร่งใสในการทำธุรกรรม” สูงกว่า 92% ซึ่งมากกว่าค่าเฉลี่ยของเว็บไซต์ทั่วไปเกือบสองเท่า สะท้อนถึงมาตรฐานการดำเนินงานที่เน้นความปลอดภัยและความน่าเชื่อถือของระบบอย่างแท้จริง
ยูฟ่าเบทปลอดภัยจริงหรือไม่?
ระบบของ ยูฟ่า ใช้มาตรการป้องกันหลายระดับ เริ่มจากการเข้ารหัส SSL Protocol, ระบบความปลอดภัยแบบ AES-256 Bit Encryption, พร้อมระบบยืนยันตัวตนด้วย OTP (One-Time Password) และระบบตรวจสอบพฤติกรรมผู้ใช้แบบ Real-Time Behavior Detection เพื่อเฝ้าระวังความผิดปกติของบัญชีอย่างต่อเนื่อง มาตรฐานเหล่านี้เทียบเท่ากับระบบความปลอดภัยที่ใช้ในสถาบันการเงินระดับโลก
ข้อมูลผู้เล่นถูกจัดเก็บอย่างไร?
ข้อมูลสำคัญทั้งหมด เช่น หมายเลขโทรศัพท์ บัญชีธนาคาร และประวัติการทำธุรกรรม จะถูกเข้ารหัสแบบ End-to-End Encryption และเก็บไว้ใน Isolated Data Vault ซึ่งเป็นระบบจัดเก็บข้อมูลแยกจากฐานข้อมูลหลัก ช่วยลดความเสี่ยงจากการเข้าถึงโดยไม่ได้รับอนุญาต และเพิ่มความปลอดภัยในระดับสูงสุด
หากเกิดปัญหาการใช้งานต้องทำอย่างไร?
สมาชิกสามารถติดต่อทีม Support ตลอด 24 ชั่วโมง ผ่าน LINE Official โดยมีเจ้าหน้าที่จริงคอยให้คำปรึกษาและช่วยเหลือทุกกรณี ไม่ว่าจะเป็นปัญหาด้านระบบ การฝาก–ถอน หรือการใช้งานทั่วไป เพื่อให้มั่นใจได้ว่าผู้เล่นทุกคนจะได้รับการดูแลอย่างมืออาชีพและต่อเนื่องตลอดเวลา
SSL Certificate ระดับ EV (Extended Validation)
เว็บไซต์ได้รับการตรวจสอบและรับรองความถูกต้องจากผู้ให้บริการด้านความปลอดภัยที่เป็นที่ยอมรับในระดับสากล เช่น DigiCert และ Sectigo
เพื่อยืนยันความปลอดภัยของข้อมูลและการเชื่อมต่อทุกครั้งที่ผู้ใช้เข้าสู่ระบบAES-256 Encryption
ระบบเข้ารหัสมาตรฐานสูงสุดที่ใช้โดยหน่วยงานด้านความมั่นคงทั่วโลก
เพื่อป้องกันการเข้าถึงข้อมูลโดยไม่ได้รับอนุญาตในทุกชั้นของการทำงานของระบบ2-Factor Authentication (2FA)
ระบบยืนยันตัวตนแบบสองขั้นตอนที่ช่วยเพิ่มความปลอดภัยของบัญชีผู้ใช้
แม้รหัสผ่านจะถูกเปิดเผย ผู้ไม่ประสงค์ดีจะไม่สามารถเข้าสู่บัญชีได้โดยง่ายAI Anti-Fraud Detection
ใช้เทคโนโลยี Machine Learning วิเคราะห์พฤติกรรมผู้ใช้แบบเรียลไทม์
เพื่อตรวจจับความผิดปกติ เช่น การล็อกอินจากหลายประเทศพร้อมกัน หรือการใช้บอทในการทำรายการ
ยูฟ่าเบทเซอร์วิส (UFA BET SERVICE) ให้ความสำคัญกับการดูแลโดยเจ้าหน้าที่จริงมากกว่าการใช้ระบบอัตโนมัติ
ทีมงานทุกคนผ่านการอบรมในด้านเทคนิค การให้คำปรึกษา และการดูแลลูกค้าอย่างมืออาชีพ
ติดต่อผ่าน LINE Official Account ที่ผ่านการยืนยันตัวตนอย่างถูกต้อง
ให้บริการตลอด 24 ชั่วโมง แบ่งการทำงานเป็น 3 กะ ไม่มีวันหยุด
มีระบบ High-Priority Case สำหรับการช่วยเหลือกรณีเร่งด่วนหรือเหตุฉุกเฉิน
ทุกการติดต่อของผู้เล่นจะได้รับการตอบกลับจากเจ้าหน้าที่จริงภายในระยะเวลาอันสั้น
เพื่อให้มั่นใจว่าการใช้งานระบบของ UFA BET เป็นไปอย่างราบรื่นต่อเนื่องทุกเวลา
Bot Detection Engine
ตรวจจับพฤติกรรมที่ผิดปกติ เช่น การกดซ้ำอย่างรวดเร็ว การเคลื่อนไหวของเมาส์ หรือจังหวะการคลิกที่ไม่เป็นธรรมชาติIP Blacklist & Geo-Fencing
ปิดกั้นการเข้าใช้งานจากพื้นที่ที่มีความเสี่ยงหรือถูกระบุว่าเป็นแหล่งการโจมตี
เพื่อป้องกันการใช้ระบบในทางที่ผิดSession Monitoring
เฝ้าระวังการเข้าสู่ระบบแบบเรียลไทม์ หากพบการล็อกอินจากหลายอุปกรณ์ในเวลาเดียวกัน
ระบบจะระงับการใช้งานชั่วคราวและแจ้งเตือนเจ้าของบัญชีทันที
ด้วยเทคโนโลยีการตรวจจับระดับสูงร่วมกับการดูแลจากทีมผู้เชี่ยวชาญตลอด 24 ชั่วโมง
ทำให้ UFABET เป็นหนึ่งในเว็บเดิมพันออนไลน์ที่ให้ความสำคัญกับความปลอดภัยและความโปร่งใสของระบบในทุกขั้นตอน
บทสรุป เว็บยูฟ่าเบท เว็บพนันครบวงจรที่รวมทุกการเดิมพันไว้ในที่เดียว
เว็บพนันยูฟ่า ถือเป็นหนึ่งในเว็บเดิมพันที่ได้รับการยอมรับในฐานะมาตรฐานของเว็บพนันครบวงจรในประเทศไทย ด้วยระบบที่รองรับการเล่นได้หลายรูปแบบ และโครงสร้างการให้บริการที่เน้น ความมั่นคง ความโปร่งใส และความปลอดภัย ในทุกขั้นตอน
จากข้อมูลการจัดอันดับของอุตสาหกรรมเกมออนไลน์ในประเทศไทย พบว่า เว็บพนัน UFA มีจำนวนผู้ใช้งานเพิ่มขึ้นเฉลี่ยปีละ 24% พร้อมอัตราการกลับมาใช้งานซ้ำ (Retention Rate) สูงถึง 68% ซึ่งสูงกว่าค่าเฉลี่ยของตลาดเกือบสองเท่า ตัวเลขเหล่านี้สะท้อนถึงคุณภาพของระบบและความเชื่อมั่นจากผู้เล่นจริงทั่วประเทศ
ยูฟ่าเบท เหมาะกับใคร?
UFA BET เหมาะสำหรับผู้เล่นที่ต้องการระบบที่ใช้งานง่าย รวมถึงนักเดิมพันที่ชอบวิเคราะห์เชิงลึก ระบบมีเครื่องมือช่วยวางแผนและคำนวณผลการเดิมพันอย่างโปร่งใส พร้อมค่าน้ำที่คุ้มค่าและโปรโมชั่นที่อัปเดตต่อเนื่อง เหมาะกับผู้ที่ต้องการสร้างผลตอบแทนระยะยาวในระบบที่มั่นคง
ยูฟ่าเบท มีเกมอะไรให้เล่นบ้าง?
เว็บยูฟ่าเบท เปิดให้บริการเกมเดิมพันครบทุกประเภทในระบบเดียว ได้แก่
แทงบอลยูฟ่าเบท ด้วยราคาน้ำที่อัปเดตแบบเรียลไทม์
คาสิโนสด ถ่ายทอดสดจากสตูดิโอมาตรฐานสากล
สล็อตออนไลน์ และ เกมยิงปลา ที่ผ่านการรับรอง RTP มาตรฐาน
หวยออนไลน์ ครบทั้งไทยและต่างประเทศ
รวมถึง กีฬาเฉพาะทาง เช่น วัวชน ไก่ชน และมวยไทย ถ่ายทอดสดจากสนามจริง
ทุกบริการถูกออกแบบให้อยู่ในระบบเดียว ผู้เล่นสามารถสลับหมวดเกมได้อย่างราบรื่นโดยไม่ต้องเปลี่ยนบัญชี
เกมครบวงจรในระบบเดียว
ยูฟ่าเบท (UFABET) รวมทุกประเภทเกมเดิมพันไว้ในระบบเดียว ไม่ว่าจะเป็น
คาสิโนสดต่างประเทศ, สล็อตออนไลน์จากค่ายชั้นนำ, แทงบอลทุกลีกทั่วโลก,
ไปจนถึง กีฬาท้องถิ่นยอดนิยม เช่น วัวชน และไก่ชน โดยไม่ต้องสลับบัญชีหรือเปลี่ยนระบบระหว่างการเล่นระบบเสถียรและใช้งานง่าย
เว็บไซต์ใช้เทคโนโลยี Progressive Web App (PWA) และโครงสร้าง Content Delivery Network (CDN) ระดับสากล
ทำให้หน้าเว็บโหลดได้รวดเร็วและใช้งานได้ลื่นไหลทั้งบนมือถือและคอมพิวเตอร์ แม้ในช่วงที่มีผู้เล่นจำนวนมากโปรโมชั่นคุ้มค่าตลอดปี
มอบความคุ้มค่าให้กับผู้เล่นด้วยกิจกรรมและโปรโมชั่นต่อเนื่อง เช่น
โบนัสสมาชิกใหม่ 100%, โบนัสเติมรายวัน, คืนค่าคอมมิชชัน 0.5% ทุกยอดเดิมพัน และกิจกรรมพิเศษประจำเดือนระบบฝาก–ถอนอัตโนมัติ
ผู้เล่นสามารถทำรายการได้ด้วยตนเองโดยไม่ต้องแจ้งสลิป ระบบรองรับทุกธนาคารในประเทศ
รวมถึง QR Code Payment ใช้เวลาเพียงประมาณ 30 วินาที ระบบจะปรับเครดิตให้อัตโนมัติทันทีทีมซัพพอร์ตดูแลตลอด 24 ชั่วโมง
มีเจ้าหน้าที่จริงให้บริการผ่าน LINE Official ตลอดวัน พร้อมให้คำแนะนำและแก้ไขปัญหาได้อย่างตรงจุด
เว็บพนันออนไลน์ยูฟ่าเบท ไม่ได้เป็นเพียงเว็บเดิมพันทั่วไป แต่พัฒนาขึ้นจากแนวคิด “เทคโนโลยีเพื่อผู้เล่น”
โดยผสานโครงสร้างระบบที่ทันสมัยเข้ากับความเข้าใจพฤติกรรมของผู้ใช้งานจริง
ทีมบริหารให้ความสำคัญทั้งด้านความเสถียรของระบบและประสบการณ์ในการใช้งานของผู้เล่นทุกกลุ่ม
ทุกบริการของ ยูฟ่าเบทออนไลน์ ถูกออกแบบให้ใช้งานง่ายโดยไม่ต้องปรับตัว ไม่ว่าจะเป็นการเล่นเพื่อความสนุกในเวลาว่าง
หรือการวางแผนเดิมพันเพื่อสร้างรายได้ในระยะยาว เว็บยูฟ่าเบท คือระบบที่รวมทุกหมวดไว้ครบในที่เดียว
ทั้งความคุ้มค่า ความปลอดภัย และความต่อเนื่องในการใช้งาน